what is the role of ethanol in dna isolation quizlet Biology videos: nucleic acid extraction
Hey guys! I stumbled upon some interesting information about ethanol and its uses in scientific experiments. I thought I would share it with you, so here it goes!
What is the freezing point of ethanol?
One of the things that caught my attention is the freezing point of ethanol. Did you know that ethanol has a freezing point of -114.1 degrees Celsius (-173.4 degrees Fahrenheit)? That’s incredibly cold! Ethanol is an alcohol that is commonly used in laboratories for various purposes, and its freezing point is quite low compared to other substances.
 Ethanol is often used as a solvent in scientific experiments due to its unique properties. It can dissolve many organic and inorganic compounds, making it an excellent choice for various laboratory procedures. In addition to being a solvent, ethanol is also used as a disinfectant, antiseptic, and even as a preservative.
Ethanol is often used as a solvent in scientific experiments due to its unique properties. It can dissolve many organic and inorganic compounds, making it an excellent choice for various laboratory procedures. In addition to being a solvent, ethanol is also used as a disinfectant, antiseptic, and even as a preservative.
The role of ethanol in a lab experiment
Another interesting aspect is the role of ethanol in lab experiments. In many cases, ethanol is used to extract and isolate DNA from biological samples. Ethanol helps in the precipitation of DNA, making it easier to separate from other components. This is particularly useful in genetic research, forensic science, and medical diagnostics.

Aside from DNA extraction, ethanol also plays a crucial role in the preservation and storage of biological samples. It helps prevent the growth of microorganisms, such as bacteria and fungi, which can degrade or contaminate the samples. Ethanol acts as a sterilizing agent, ensuring the integrity and quality of the samples.
In conclusion, ethanol is not just your average alcoholic drink. It has remarkable properties that make it an indispensable component in many scientific experiments. Its low freezing point, solvency, and DNA-precipitating abilities make it a valuable tool in laboratories all over the world. So, the next time you come across a bottle of ethanol, remember its significant role in scientific research!
If you are looking for Biology Videos: Nucleic Acid Extraction - DNA isolation you’ve visit to the right place. We have 5 Pictures about Biology Videos: Nucleic Acid Extraction - DNA isolation like Solved What is the role of ethanol in this lab? How the | Chegg.com, Biology Videos: Nucleic Acid Extraction - DNA isolation and also Ethanol | Anhydrous Alcohol | RNA & DNA Extraction | IBI Scientific. Here it is:
Biology Videos: Nucleic Acid Extraction - DNA Isolation
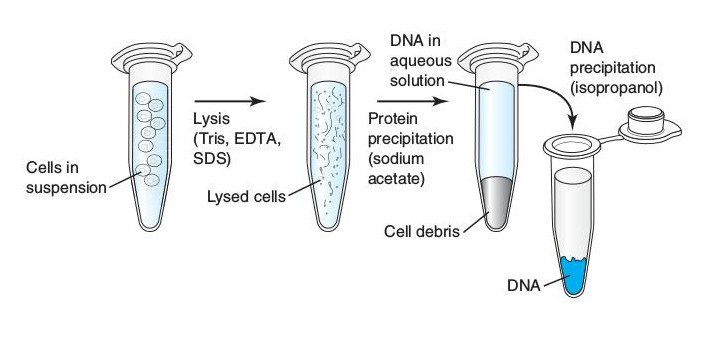 biolvid.blogspot.comdna isolation extraction nucleic acid organic general scheme
biolvid.blogspot.comdna isolation extraction nucleic acid organic general scheme
Ethanol | Anhydrous Alcohol | RNA & DNA Extraction | IBI Scientific
 www.ibisci.comethanol anhydrous extraction
www.ibisci.comethanol anhydrous extraction
What Is The Freezing Point Of Ethanol? - ScienceNote.info
 sciencenote.infofreezing ethanol
sciencenote.infofreezing ethanol
Solved What Is The Role Of Ethanol In This Lab? How The | Chegg.com
 www.chegg.comCloning And Genetic Engineering · Concepts Of Biology
www.chegg.comCloning And Genetic Engineering · Concepts Of Biology
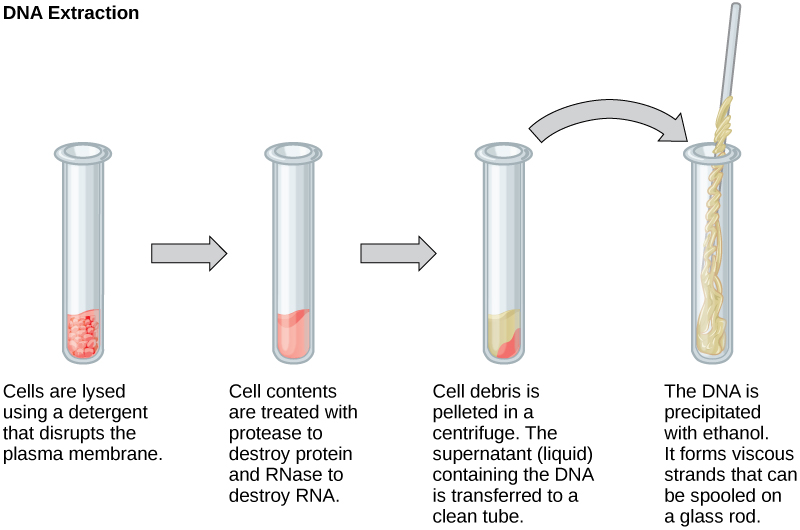 philschatz.comcells biology dna rna cell test tube tubes genetic protein cloning glass four extraction steps ethanol method diagram extracting gene
philschatz.comcells biology dna rna cell test tube tubes genetic protein cloning glass four extraction steps ethanol method diagram extracting gene
Freezing ethanol. Solved what is the role of ethanol in this lab? how the. Cells biology dna rna cell test tube tubes genetic protein cloning glass four extraction steps ethanol method diagram extracting gene